Two common hospital superbugs have been shown to work together to resist treatment, in a new study led by Macquarie University. The researchers discovered Klebsiella pneumoniae and Acinetobacter baumannii share food and protect each other from antibiotics.

Ganging up: Researchers Associate Professor Amy Cain (pictured left) and Dr Lucie Semenec (pictured right) showed common hospital superbugs work together in resisting drugs.
The study, published in Nature Communications, suggests hospitals need to modify screening methods for drug-resistant bacteria. It also offers a path to improved treatments to fight hospital-acquired infections.
“If you have a long hospital stay, you have a high risk of being infected by drug-resistant bacteria,” says Macquarie University researcher Dr Lucie Semenec, lead author of the study.
“Currently, clinical diagnostic methods focus on the single most dominant infecting pathogen and are treated based on this information.
“However, it’s important to realise there is an added layer of complexity where other co-infecting pathogens may be affecting the resistance and virulence profile of the infections.”
This is foundational knowledge that could be a game changer.
Studies in the US and Europe have found the two superbugs living together in about 40 per cent of all hospitalised patients.
“We have identified how K. pneumoniae and A. baumannii work together,” says Semenec.
“We discovered how Klebsiella feeds Acinetobacter through its metabolic by-products. And, in return, we found that Acinetobacter protects Klebsiella from high concentrations of drugs through antibiotic-degrading enzymes it secretes.
“This mutually beneficial relationship enables Klebsiella to survive in antibiotic concentrations significantly higher than it can on its own.”
Co-lead author, Macquarie University’s Associate Professor Amy Cain, says the research highlights the pressing need for improved screening for mixed infections in hospital settings.
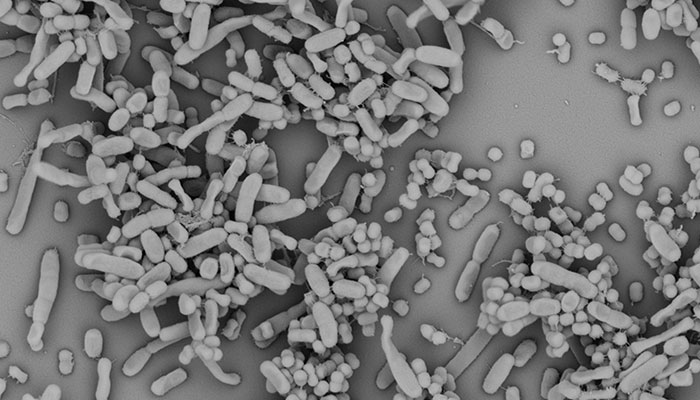
Under the microscope: Image showing Klebsiella pneumoniae and Acinetobacter baumannii growing together. Image credit: Dr Lucie Semenec.
“It’s important to understand that together, these bugs are more infectious, more resistant to treatment and they feed off each other,” says Cain.
“This is foundational knowledge that could be a game changer.
“It explains why we can’t just blast one of the bugs. Now we are going to see if we can disrupt the collaboration between these two bacteria to get them into a more treatable condition.”
A decade of collaboration
The study investigated two strains previously co-isolated from a single human lung infection and examined them using multiple screening and analysis mechanisms, from microscopy to genomics and infections in living organisms.
The researchers used caterpillars as an ethical animal model. The larvae of the greater wax moth, Galleria mellonella, usually live in beehives in the tropics where they largely live on beeswax.
The caterpillars grow well at human body temperature making them ideal to study bacteria that grow in the body. The Macquarie Galleria Research Facility, where the studies were conducted, is the first of its kind in Australia.
This week’s published research is the result of more than a decade of collaboration between three research groups: Dr Cain’s group at Macquarie University, Professor Ian Paulsen’s group also at Macquarie University, and Associate Professor Karl Hassan’s group at the University of Newcastle.
"Rather like photographing a sculpture from different angles so you can see it its entirety, we really needed a combination of methods to understand this interaction,” says Semenec.
- Non-certified surgeons to blame for many breast implant complications: study
- VIDEO: Education rethink as ChatGPT hits classrooms
Together, they have used increasingly more sophisticated technologies to push forward their understanding of the interactions within microbial communities.
Researchers say the work has implications not just for the fight against drug resistant superbugs, but also helps explain how bacteria can work together to form sticky layers, known as biofilms, on catheters and other medical devices.
It is also important for the new field of synthetic biology. The ARC Centre of Excellence in Synthetic Biology is headquartered at Macquarie University and is working to design and build communities of microbes that could to turn agricultural and municipal waste into a vast array of sustainable products.
Dr Amy Cain is an ARC Future Fellow in the School of Natural Sciences, Macquarie University.
Dr Lucie Semenec is a Postdoctorate Research Fellow in Bacterial Genetics in the School of Natural Sciences, Macquarie University.
Read more about the Macquarie University Galleria Research Facility