A team of Macquarie University neurosurgery and computer science researchers is investigating the use of Artificial Intelligence and other computer tools to improve the study of brain disease in the University’s world-first Computational NeuroSurgery (CNS) Laboratory, with results already indicating far-reaching impact for disease diagnosis and treatment.
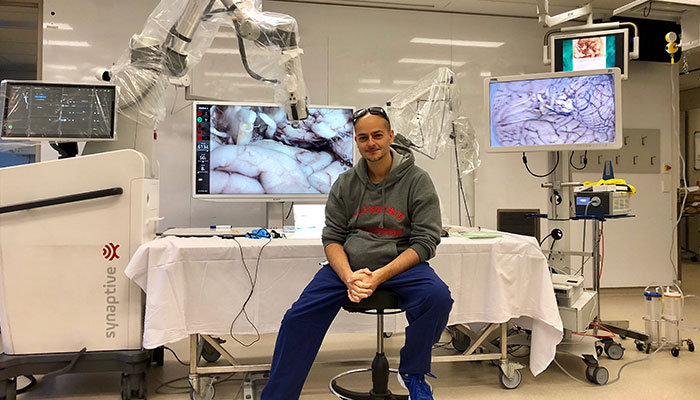
Innovation: Associate Professor Antonio Di leva, pictured, and his team have pioneered the use of AI-driven technology to improve the accuracy of brain images and in turn improve patient diagnosis and treatment.
The CNS Lab which was recently founded by Macquarie University Associate Professor Antonio Di Ieva, who is also a practising neurosurgeon at Macquarie University Hospital, is focused on developing computerised tools to produce more accurate images of the brain.
“Our research is to develop new computer methods to identify novel diagnostic, prognostic and therapeutic markers of brain diseases, such as brain tumours and cerebrovascular diseases ,” Associate Professor Di Ieva says.
In their latest research, lead by Di Ieva and collaborator, Dr Sidong Liu, an NHMRC Early Career Fellow from the Australian Institute of Health Innovation, and in partnership with several centres at Macquarie University and overseas (Universities of Istanbul and Doha), the team used an AI method called Deep Learning to analyse surgical samples of gliomas and to predict patient outcome and treatment.
The use of Deep Learning to analyse surgical samples allows a much faster and cheaper way to predict the presence of important markers, improving and speeding up the treatment of patients affected by brain cancer.
The research looked at patients affected by gliomas –the most common primary brain tumours.
Among this diverse category of brain tumours, glioblastoma is the most malignant one, with about 1000 new cases every year in Australia, and with only five per cent of patients surviving five years after the diagnosis. When doctors find a particular mutation in the DNA, in the so-called IDH gene, it allows them to confirm the diagnosis of a type of glioma which has a better prognosis.
Trials are currently in progress worldwide to use this mutation as a target for specific vaccines, opening new avenues in the treatment of brain cancer. The current gold standard to identify such an important mutation is by performing a brain tumour biopsy and analysing the sample, which requires money and time (up to a few weeks).
Associate Professor Di Ieva and Macquarie Medical Imaging are the first ones to introduce in Australia a Magnetic Resonance technique called 2HG-Magnetic Resonance Spectroscopy to predict the status of such a gene even before surgery.
- World-first study at sea reveals COVID's silent menace
- 75 years of the UN - its triumphs and disasters
Moreover, the use of Deep Learning to analyse surgical samples of gliomas allows a much faster and cheaper way to predict the presence of this important marker, improving and speeding up the treatment of patients affected by brain cancer.
The researchers have also pioneered a way to harness an AI technique, called Generative Adversarial Network (GAN), to produce “fake gliomas”, which are tumours that appear to be like gliomas but are not actually from patients. By doing this, the computer can be trained in a few hours at the same level, or even better, than a pathologist who reaches that expertise after several years of training. This is a significant achievement with positive impacts for patients in the future.
Liu, Di Ieva et al. published their findings in Scientific Reports, the research journal of Nature.
Technology does not replace the role of clinicians
When it comes to brain disease, diagnostic errors lead doctors toward incorrect decision-making and over- or under-treating disease can be the consequence.
Nowadays, in order to limit errors and find agreement on patient treatment plans, the implementation of multi-disciplinary teams has significantly helped to increase the accuracy of diagnosis, estimates of long-term outcomes and enhanced decision-making around whether or not to proceed to surgery, although this is still far from perfect for finding a standardised manner to treat patients.
Aware of these limitations, Associate Professor Di Ieva used his knowledge of computer science and network of engineers and mathematicians to tackle brain diseases in a different way.
He was the first neurosurgeon in the world who applied a mathematical model called fractal geometry, a field of maths introduced in the 1970s to explain the complexity of Nature, to quantify the complexity of the brain and its diseases in a more precise way.
He started by applying the mathematical model to the study of tumours of the pituitary gland and discovered a subset of tumours, that are generally considered to be benign, behave in a more aggressive way, even resembling cancer in some cases. This was the beginning of a paradigm shift that brought him and several collaborators across the world to study brain diseases through computational modelling. (Summarised in the only textbook in the field, The Fractal Geometry of the Brain, Springer, New York, 2016).
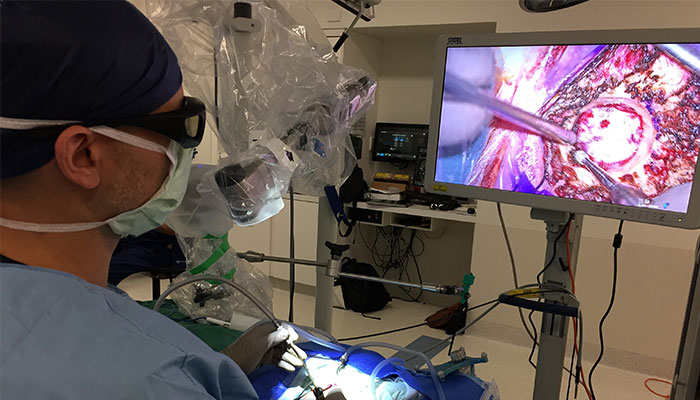
A new discipline: Prof Di Ieva used computers to study brain tumours using ‘computational neurosurgery’, which has recently advanced even further by the application of Artificial Intelligence (AI).
“This information can be used to implement the decision-making from the multi-disciplinary team meetings, where the expert decisions can be enhanced by the power of machines,” Associate Professor Di Ieva says.
“The long-term goal is to enhance treatment and outcomes for patients.”
The first stage of work began in the last year, with researchers mapping the ‘fingerprint’ of the brain – the highly detailed architecture of the normal or diseased brain.
“The aim is to support, not to replace, clinicians (and other experts),” Di Ieva said in a letter to medical journal The Lancet.
Associate Professor Di Ieva, who has been awarded an Australian Research Council (ARC) future fellowship grant, is an Associate Professor of neurosurgery at Macquarie University Hospital.



